Chemical Potential¶
In this tutorial we learn how to generate a basic bulk phase diagram from DFT energies. This enables the comparison of the thermodynamic stability of various different bulk phases under different chemical potentials giving valuable insight in to the syntheis of solid phases. This example will consider a series of bulk phases which can be defined through a reaction scheme across all phases, thus for this example including Bulk, 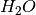 and
and 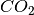 as reactions and A as a generic product.
as reactions and A as a generic product.
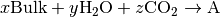
The system is in equilibrium when the chemical potentials of the reactants and product are equal; i.e. the change in Gibbs free energy is 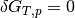 .
.
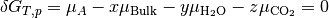
Assuming that 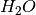 and
and 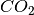 are gaseous species,
are gaseous species, 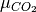 and
and 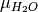 can be written as
can be written as
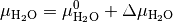
and
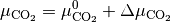
The chemical potential 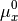 is the partial molar free energy of any reactants or products (x) in their standard states, in this example we assume all solid components can be expressed as
is the partial molar free energy of any reactants or products (x) in their standard states, in this example we assume all solid components can be expressed as
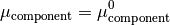
Hence, we can now rearrange the equations to produce;
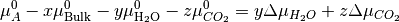
As 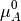 corresponds to the partial molar free energy of product A, we can replace the left side with the Gibbs free energy (
corresponds to the partial molar free energy of product A, we can replace the left side with the Gibbs free energy (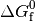 ).
).
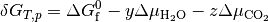
At equilibrium 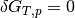 , and hence
, and hence
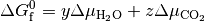
Thus, we can find the values of 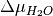 and
and 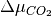 (or
(or 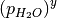 and
and 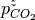 when solid phases are in thermodynamic equilibrium; i.e. they are more or less stable than Bulk. This procedure can then be applied to all phases to identify which is the most stable, provided that the free energy
when solid phases are in thermodynamic equilibrium; i.e. they are more or less stable than Bulk. This procedure can then be applied to all phases to identify which is the most stable, provided that the free energy 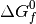 is known for each solid phase.
is known for each solid phase.
The free energy can be calculated using

Where for this tutorial the free energy (G) is equal to the calculated DFT energy (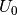 ).
).
import matplotlib.pyplot as plt
from surfinpy import bulk_mu_vs_mu as bmvm
from surfinpy import utils as ut
from surfinpy import data
The first thing to do is input the data that we have generated from our DFT simulations. The input data needs to be contained within a class. First we have created the class for the bulk data, where ‘Cation’ is the number of cations, ‘Anion’ is the number of anions, ‘Energy’ is the DFT energy and ‘F-Units’ is the number of formula units.
bulk = data.ReferenceDataSet(cation = 1, anion = 1, energy = -92.0, funits = 10)
Next we create the bulk phases classes - one for each phase. ‘Cation’ is the number of cations, ‘x’ is in this case the number of water species (corresponding to the X axis of the phase diagram), ‘y’ is the number of in this case 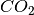 molecules (corresponding to the Y axis of our phase diagram), ‘Energy’ is the DFT energy and finally ‘Label’ is the label for the phase (appears on the phase diagram).
molecules (corresponding to the Y axis of our phase diagram), ‘Energy’ is the DFT energy and finally ‘Label’ is the label for the phase (appears on the phase diagram).
Bulk = data.DataSet(cation = 10, x = 0, y = 0, energy = -92.0, label = "Bulk")
A = data.DataSet(cation = 10, x = 5, y = 20, energy = -468.0, label = "A")
B = data.DataSet(cation = 10, x = 0, y = 10, energy = -228.0, label = "B")
C = data.DataSet(cation = 10, x = 10, y = 30, energy = -706.0, label = "C")
D = data.DataSet(cation = 10, x = 10, y = 0, energy = -310.0, label = "D")
E = data.DataSet(cation = 10, x = 10, y = 50, energy = -972.0, label = "E")
F = data.DataSet(cation = 10, x = 8, y = 10, energy = -398.0, label = "F")
Next we need to create a list of our data. Don’t worry about the order, surfinpy will sort that out for you.
data = [Bulk, A, B, C, D, E, F]
We now need to generate our X and Y axis, or more appropriately, our chemical potential values. These exist in a dictionary. ‘Range’ corresponds to the range of chemcial potential values to be considered and ‘Label’ is the axis label. Additionally, the x and y energy need to be specified.
deltaX = {'Range': Range of Chemical Potential,
'Label': Species Label}
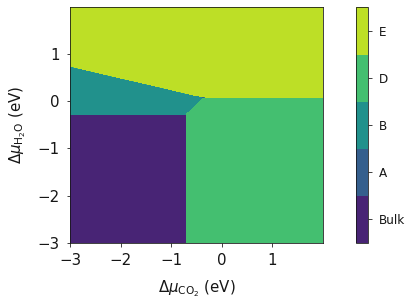
deltaX = {'Range': [ -3, 2], 'Label': 'CO_2'}
deltaY = {'Range': [ -3, 2], 'Label': 'H_2O'}
x_energy=-20.53412969
y_energy=-12.83725889
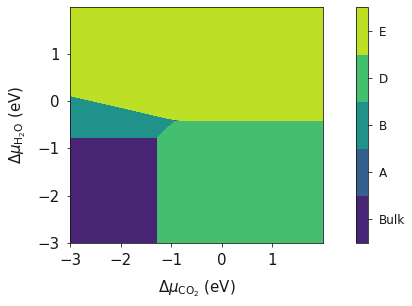
And finally we can generate our plot using these 6 variables of data.
system = bmvm.calculate(data, bulk, deltaX, deltaY, x_energy, y_energy)
ax = system.plot_phase()
plt.show()
Temperature¶
In the previous example we generated a phase diagram at 0 K. However, this is not representative of normal conditions.
Temperature is an important consideration for materials chemistry and we may wish to evaluate the phase thermodynamic stability at various synthesis conditions.
This example will again be using the 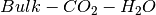 system.
system.
As before the free energy can be calculated using;

Where for this tutorial the free energy (G) for solid phases is equal to is equal to the calculated DFT energy 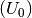 .
For gaseous species, the standard free energy varies significantly with temperature, and as DFT simulations are designed for condensed phase systems,
we use experimental data to determine the temperature dependent free energy term for gaseous species,
where
.
For gaseous species, the standard free energy varies significantly with temperature, and as DFT simulations are designed for condensed phase systems,
we use experimental data to determine the temperature dependent free energy term for gaseous species,
where 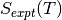 is specific entropy value for a given T and
is specific entropy value for a given T and 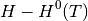 is the , both can be obtained from the NIST database and can be calculated as;
is the , both can be obtained from the NIST database and can be calculated as;
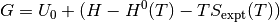
from surfinpy import bulk_mu_vs_mu as bmvm
from surfinpy import utils as ut
from surfinpy import data
bulk = data.ReferenceDataSet(cation = 1, anion = 1, energy = -92.0, funits = 10)
Bulk = data.DataSet(cation = 10, x = 0, y = 0, energy = -92.0, label = "Bulk")
A = data.DataSet(cation = 10, x = 5, y = 20, energy = -468.0, label = "A")
B = data.DataSet(cation = 10, x = 0, y = 10, energy = -228.0, label = "B")
C = data.DataSet(cation = 10, x = 10, y = 30, energy = -706.0, label = "C")
D = data.DataSet(cation = 10, x = 10, y = 0, energy = -310.0, label = "D")
E = data.DataSet(cation = 10, x = 10, y = 50, energy = -972.0, label = "E")
F = data.DataSet(cation = 10, x = 8, y = 10, energy = -398.0, label = "F")
data = [Bulk, A, B, C, D, E, F]
x_energy=-20.53412969
y_energy=-12.83725889
In order to calculate 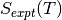 for
for 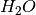 and
and 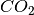 we need to use experimental data from the NSIT JANAF database.
As a user you will need to download the tables for the species you are interested in (in this example water and carbon dioxide).
surfinpy has a function that can read this data, assuming it is in the correct format and calculate the temperature correction for you.
Provide the path to the file and the temperature you want.
we need to use experimental data from the NSIT JANAF database.
As a user you will need to download the tables for the species you are interested in (in this example water and carbon dioxide).
surfinpy has a function that can read this data, assuming it is in the correct format and calculate the temperature correction for you.
Provide the path to the file and the temperature you want.
CO2_exp = ut.fit_nist("CO2.txt")[298]
Water_exp = ut.fit_nist("H2O.txt")[298]
CO2_corrected = x_energy + CO2_exp
Water_corrected = y_energy + Water_exp
deltaX = {'Range': [ -3, 2], 'Label': 'CO_2'}
deltaY = {'Range': [ -3, 2], 'Label': 'H_2O'}
CO2_corrected and H2O_corrected are now temperature depenent terms correcsponding to a temperature of 298 K. The resulting phase diagram will now be at a temperature of 298 K.
system = bmvm.calculate(data, bulk, deltaX, deltaY, x_energy=CO2_corrected, y_energy=Water_corrected)
system.plot_phase(temperature=298)
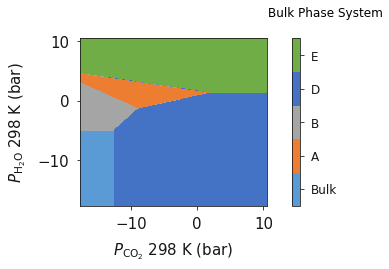
Pressure¶
In the previous example we went through the process of generating a simple phase diagram for bulk phases and introducing temperature dependence for gaseous species. This useful however, sometimes it can be more beneficial to convert the chemical potenials (eVs) to partial presure (bar).
Chemical potential can be converted to pressure values using
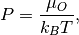
where P is the pressure, 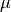 is the chemical potential of oxygen, $k_B$ is the Boltzmnann constant and T is the temperature.
is the chemical potential of oxygen, $k_B$ is the Boltzmnann constant and T is the temperature.
import matplotlib.pyplot as plt
from surfinpy import bulk_mu_vs_mu as bmvm
from surfinpy import utils as ut
from surfinpy import data
colors = ['#5B9BD5', '#4472C4', '#A5A5A5', '#772C24', '#ED7D31', '#FFC000', '#70AD47']
Additionally, surfinpy has the functionality to allow you to choose which colours are used for each phase. Specify within the DataSet class color.
bulk = data.ReferenceDataSet(cation = 1, anion = 1, energy = -92.0, funits = 10)
Bulk = data.DataSet(cation = 10, x = 0, y = 0, energy = -92.0, color=colors[0], label = "Bulk")
A = data.DataSet(cation = 10, x = 10, y = 0, energy = -310.0, color=colors[1], label = "A")
B = data.DataSet(cation = 10, x = 0, y = 10, energy = -228.0, color=colors[2], label = "B")
C = data.DataSet(cation = 10, x = 8, y = 10, energy = -398.0, color=colors[3], label = "C")
D = data.DataSet(cation = 10, x = 5, y = 20, energy = -468.0, color=colors[4], label = "D")
E = data.DataSet(cation = 10, x = 10, y = 30, energy = -706.0, color=colors[5], label = "E")
F = data.DataSet(cation = 10, x = 10, y = 50, energy = -972.0, color=colors[6], label = "F")
data = [Bulk, A, B, C, D, E, F]
x_energy=-20.53412969
y_energy=-12.83725889
CO2_exp = ut.fit_nist("CO2.txt")[298]
Water_exp = ut.fit_nist("H2O.txt")[298]
CO2_corrected = x_energy + CO2_exp
Water_corrected = y_energy + Water_exp
deltaX = {'Range': [ -1, 0.6], 'Label': 'CO_2'}
deltaY = {'Range': [ -1, 0.6], 'Label': 'H_2O'}
system = bmvm.calculate(data, bulk, deltaX, deltaY, x_energy=CO2_corrected, y_energy=Water_corrected)
system.plot_phase()
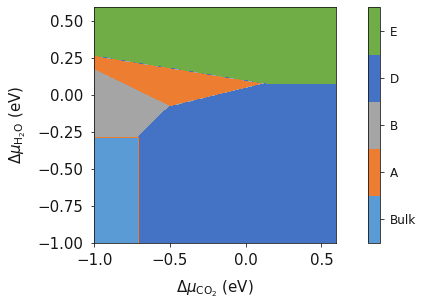
To convert chemical potential to pressure use the plot_pressure command and the temperature at which the pressure is calculated. For this example we have used 298 K.
system.plot_pressure(temperature=298)